
solvent
explicit
-
atomistic detail (many degrees of freedom)
-
expensive (many molecules, solvent relaxation)
-
solute-solvent interactions
-
samples smaller portion of phase space
implicit
-
mean-field detail (PB equation, GB approach)
-
inexpensive (no solvent degrees of freedom)
-
misses some solute-solvent features (e.g. hydrogen bonds)
-
samples bigger portion of phase space
monte carlo
-
imagine two states, X and Y
-
similar to chemical kinetics
-
detailed balance
-
transition probability
monte carlo
-
equal to opposite move
-
Boltzmann distribution
-
metropolis choice (satisfies condition)
-
equivalent to
pH thermodynamics
-
Henderson Hasselbach
-
pH thermodynamics
-
MD people use protonation fraction
-
interactions cause deviations

Last note on pH
-
protonated fraction in terms of free energy
-
by comparing with
-
we get
Constant pH Molecular Dynamics (CpHMD)
-
md samples configurations under constant protonation states
-
we want to sample configurations under constant pH
-
also we want titration curve to calculate pKa and Hill coefficient
-
hybrid method, leverages monte carlo and classical molecular dynamics
-
The key quantity is the free energy of protonation
(Amber
manual
p. 563)
free energy of protonation
-
no knowledge of
-
need to use reference compound
-
thermodynamic cycle
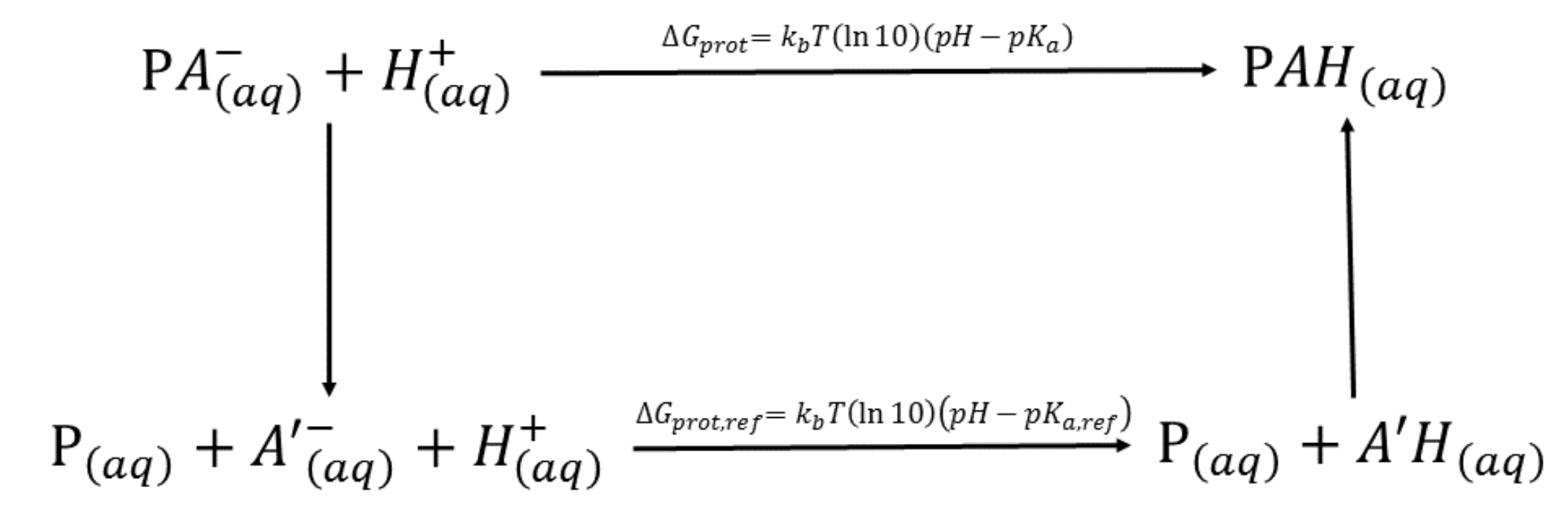
(Vinny's dissertation)
free energy of protonation

-
-
AMBER approximation
sampling of protonation states
-
within a few lines of algebra
-
)
-
MC move
-
MD/MC will sample entire protonation space, ergodicity
-
get
-
multiple
